Abstract
Multiparametric flow cytometry (MPFC) is a mainstream laboratory method used in the diagnosis of multiple myeloma. Minimal residual disease (MRD) assessment by EuroFlow next-generation flow cytometry allows assessment down to an assay sensitivity of 1x10 -5.
Delayed sample processing remains a common challenge due to logistical limitations. Specialized tests performed in central pathology laboratories are frequently located a considerable distance from healthcare providers.
Our study aims to evaluate the impact of delayed sample processing on plasma cell yield and bone marrow sample stability. There is little published data available.
Plasma cell yield and bone marrow sample stability were investigated in patients with multiple myeloma who underwent bone marrow biopsy. Participants were included based on ³10% plasma cell burden by morphological quantification on the bone marrow aspirate smear. Bone marrow aspirates were collected in EDTA (with three samples also collected in lithium heparin) and stored at four degrees Celsius. Samples were analyzed by MPFC within four hours of collection, at 24 and at 48 hours after collection. CD138 and CD38 co-expression were used to identify plasma cells, and absence of 7-AAD to determine cell viability. Mean fluorescence intensity (MFI) of CD138 and CD38 was recorded. Statistical analyses were performed using two-tailed Wilcoxon signed-rank tests and repeated measures ANOVA with significance assigned at p<0.05.
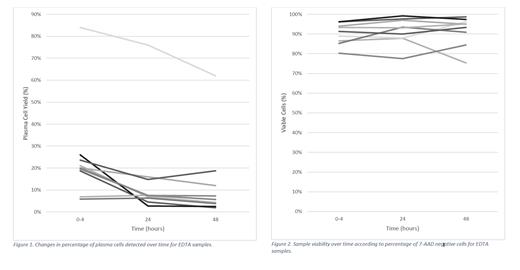
Bone marrow aspirate samples of nine participants were evaluated. Significant reduction in plasma cell yield was observed over time (p<0.001) while sample integrity remained unchanged (p>0.05). The most marked reduction in plasma cell detection was seen between initial processing and 24 hours (median absolute reduction 9%, range 0 to 23% and median relative reduction 37%, range -8 to 90%, p<0.01). Further significant reduction of plasma cells occurred after an additional 24 hours (p=0.025). At 48 hours, the median absolute reduction in plasma cell yield from initial testing was 12% (range 1 to 24%) and median relative reduction was 40% (range 18 to 90%).
Sample integrity remained constant. The median viability at collection, 24 hours and 48 hours was 91%, 93% and 95% respectively. The most significant specimen deterioration observed was 13% viability reduction to 75% overall by 48 hours.
Three of the participants had additional samples collected in lithium heparin anticoagulant media that were analyzed in parallel with their EDTA samples. Plasma cell yield remained similar across the two different anticoagulants with overall cell viability remaining high in lithium heparin (³90%). A trend of time-dependent reduction of CD138 MFI was observed with lithium heparin but not with EDTA.
This study demonstrates the significance of time to processing as a pre-analytical variable in MPFC in multiple myeloma. The greatest loss of plasma cells occurs within the first 24 hours after collection but continues to fall significantly out to 48 hours. Reductions of up to 90% were observed in our small cohort and represent a potential 1 log reduction in yield. This decrease in plasma cell yield raises questions of reliability and validity of flow cytometry, whereby the sensitivity depth may be compromised if the sample cannot be processed on the same day of collection. It is a technical limitation of flow cytometry in comparison to polymerase chain reaction methods where sensitivity is unaffected by delays in processing.
The overall viability of cells within the samples remained stable over time, despite the decline in plasma cells. A reduction in CD138 MFI is observed in lithium heparin storage medium that may impact on standardized gating techniques. Further validation studies are warranted to explore these phenomena.
MRD monitoring in multiple myeloma is rapidly becoming an accepted standard of care in the evaluation of treatment response and represents an independent prognostic maker of progression free survival that can be used to guide further therapy. Our findings indicate the potential of false negative MRD results with delays in sample processing. This questions the current consensus guidelines that recommend samples can be processed up to 2 days after collection. These guidelines may need to be revised in the near future.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal